Abstract
Delayed T cell reconstitution after allogeneic hematopoietic stem cell transplant (allo-HSCT) is an important contributor to transplant-related morbidity and mortality due to infection and malignant relapse. Optimal T cell recovery requires a functional thymus, and strategies to enhance T cell reconstitution have the potential to improve overall outcome in allo-HSCT recipients, however, at the present time such strategies are limited. Hence one of the most significant clinical challenges is the need for rapid regeneration of thymopoiesis following induced immunodepletion and transplantation.
Zinc is the second most abundant trace metal in the body, binding to more than 300 proteins involved in DNA synthesis and repair, gene transcription, cell proliferation as well as differentiation and apoptosis. Zinc deficiency (ZD) is a clinical condition causing immunosuppression and thymic atrophy with a consequent reduction in the number of circulating recent thymic emigrants (RTEs). Furthermore, mild ZD is one of the causes of the reduction in thymic function in the elderly and the role of zinc in tissue regeneration after damage has been clearly demonstrated in liver, skin, and intestinal diseases. In a pilot clinical trial, we demonstrated that patients receiving oral zinc supplementation after autologous HSCT showed increased thymic-dependent T cell reconstitution in the absence of adverse clinical events (Iovino 2018, Leuk Res). Although a clear clinical benefit was observed, the mechanisms underlying this process are poorly understood. Thus, we used a murine model to evaluate the effect of zinc supplementation in thymic reconstitution after acute damage.
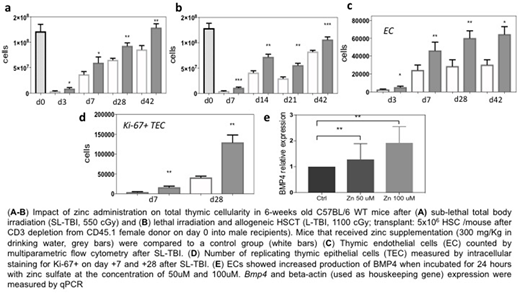
Using a model of thymic damage caused by sub-lethal total body irradiation (SL-TBI, 550 cGy), we found that mice that received zinc supplementation demonstrated increased thymic cellularity when compared to untreated age-matched mice (Fig. 1a). Importantly, this finding was also confirmed in a clinically-applicable model of MHC-matched allogeneic HSCT (Fig. 1b). We have previously demonstrated endothelial cells (EC), which are extremely resistant to damaged, are able to trigger thymic endogenous reconstitution after damage by producing regenerative factors such as BMP4, which targets thymic epithelial cells (TECs), a key population crucial for T cell development (Wertheimer 2018, Science Immunol). Interestingly, in our model of zinc administration, we found an increase in the number of regeneration-initiating ECs (Fig. 1c), and increased proliferation of TECs (Fig. 1d), which can occur in response to BMP4. Consistent with the hypothesis that zinc supplementation is activating the BMP4 pathway, when stimulated in vitro for 24 hours with supraphysiological doses of zinc sulfate, ex vivo propagated ECs (exECs) were directly induced to produce BMP4 (Fig. 1e), suggesting a likely mechanism by which zinc supplementation promotes thymic reconstitution.
In conclusion, we demonstrate a mechanism by which zinc supplementation can improve thymic function and offers an innovative therapeutic strategy to improve T cell reconstitution in patients receiving allo-HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal